Megan Williams is Associate Professor of Neurobiology and Anatomy. Her lab is working toward cracking the molecular code of synapse specificity. They have identified several cell adhesion molecules necessary for the form and function of highly specific types of synapses that, when disrupted, may lead to neurodevelopmental disorders like autism and intellectual disability.
Research Overview:
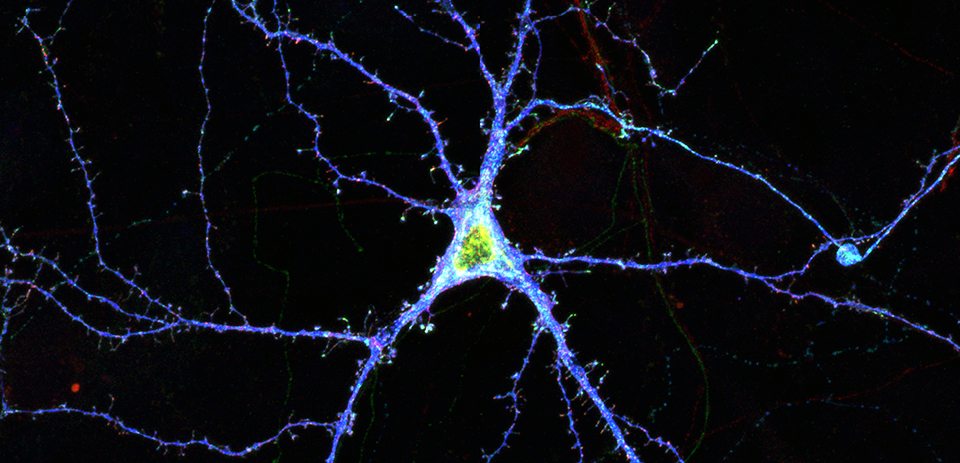
Research in the Williams lab seeks to elucidate mechanisms regulating target-specific synapse formation during brain development. The brain contains billions of neurons that form trillions of synapses. Importantly, the brain is not wired up randomly. Instead, proper brain function requires that neurons build specific types of synapses with distinct target neurons while avoiding incorrect targets. Moreover, it is often overlooked that synapses are not all alike but rather synapses between different types of neurons acquire very different structural, functional, and molecular properties. Being able to activate, silence, and manipulate specific classes of synapses, not just cell types, is one of the next big challenges in cellular neuroscience and the Williams lab is taking bold steps to tackle this problem in the mammalian brain. Remarkably, all key synapse specificity molecules identified by the Williams lab thus far, including the cell adhesion proteins Kirrel3 and the classic cadherins, are associated with autism and intellectual disabilities in human genetic studies. Thus, work from the Williams lab is poised to shed light on the basic cellular defects underlying some neurodevelopmental disorders and facilitate the development of synapse-specific treatments with fewer side effects. To accomplish their goals, the lab uses a variety of genetic and CRISPR/Cas9 gene editing tools, light and electron microscopy, and electrophysiology.
Recent Highlights from the Williams lab:
- Hot off the press! Previously, the Williams lab discovered that Kirrel3 is necessary to form a specific type of synapse in the hippocampus, a key brain region for learning and memory. Variants in the human Kirrel3 gene are repeatedly identified as risk factors for autism and intellectual disability. Recently, the lab demonstrated that many disease-associated Kirrel3 missense variants have impaired synaptogenic function in cultured neurons. This is the first functional evidence that Kirrel3 variants may lead to brain disorders and was recently published in the Journal of Neuroscience.
- Student Success: Williams lab member Arnulfo “Tuna” Tunon-Ortiz earned a spot on the NIH-sponsored Developmental Biology Training Grant and his inspiring scientific journey is featured in this video.
- Matthew Taylor successfully defended his Ph.D thesis on his research in the Williams lab and is off to pursue postdoctoral research at UC Denver.
- Check out the Williams Lab website for more information and publications.
Training and Mentorship:
Dr. Williams’s trainees have consistently won local and national awards including an Autism Speaks Fellowship, University-wide Best Dissertation award, and NIH Training grant fellowships. She is active in graduate (Developmental Neuroscience) and medical teaching (Histology lab and case-based learning). She also holds many other mentoring and leadership roles including chair of advising for the Neuroscience PhD program, president of the Intermountain chapter of the Society for Neuroscience, and member of the directorates for the Interdepartmental program in Neuroscience and Department of Neurobiology and Anatomy PhD programs.